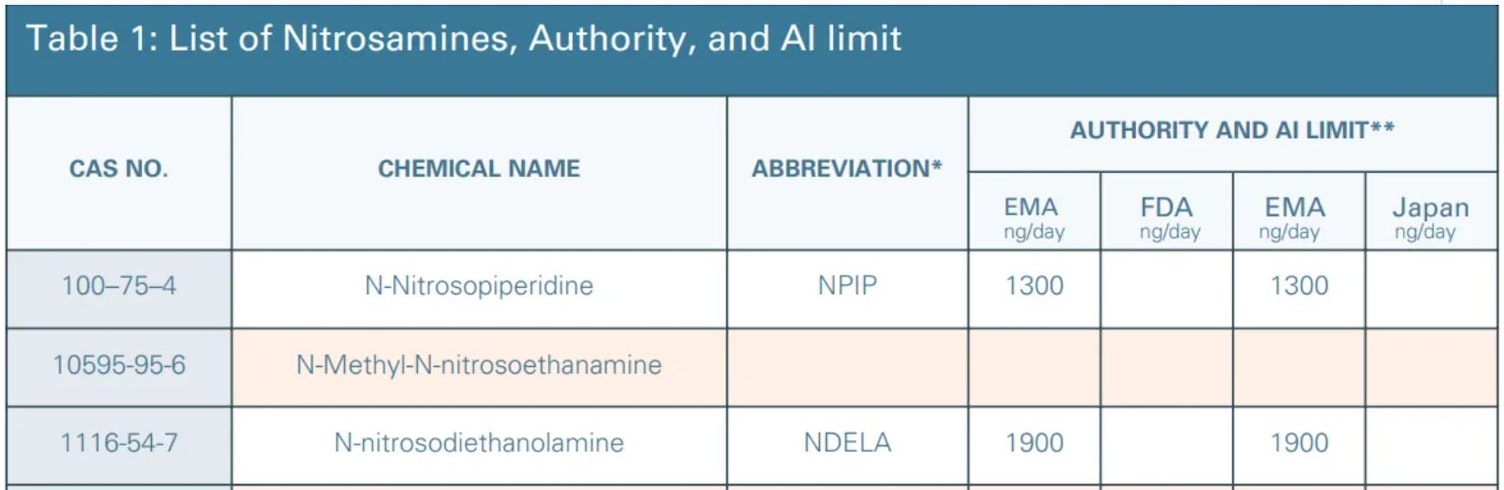
A risk-based approach
All guidance documents included the demand for a risk-based approach to detecting the potential presence of nitrosamines or for nitrosation of their drug compounds. Some risks include the presence or potential for:
- Secondary, tertiary, or quaternary amines in the product, either as an active drug compound, excipient, or a synthetic/degradant impurity. An acidic environment or microenvironment in solid dose products to help potentially support the reaction if other ingredients are present and conditions are suitable for the generation of nitrosamines.
- Nitrite or nitrate that may have been present as a reagent in synthesis and present in an oxidative environment to help the formation of nitrosamines.
- Certain organic solvents, including amides, which may either contain secondary amine impurities or form them upon thermal degradation, reacting with commonly used nitrous acid to form nitrosamines. Tertiary and quaternary amine reagents are commonly used in synthesis which can potentially be converted to nitrosamines.