Introduction
As a Notified Body (NB 1639), we are designated under the EU IVDR to carry out conformity assessment and certification of in vitro diagnostic medical devices. Once certified with us, you can affix the CE 1639 mark to your IVD medical devices and place them on the EU market.
Our designation covers the full spectrum of IVD clinical domains and technologies. We can certify:
- Class A sterile devices
- Class B devices
- Class C devices
Devices belonging to risk Class D and Companion Diagnostics are currently outside of our designation scope.
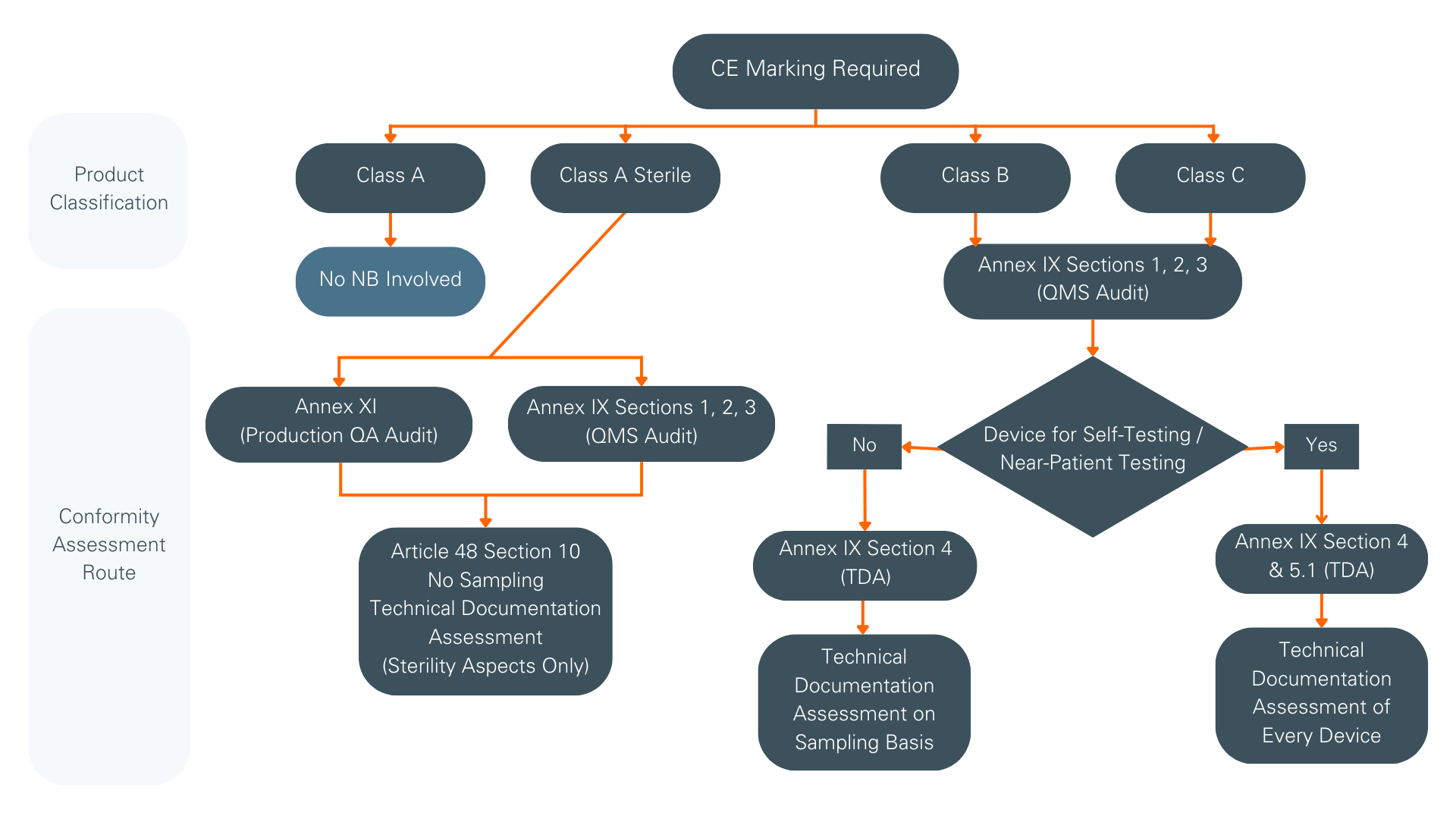
Upon successful completion of the Quality Management System (QMS) audit and Technical Documentation Assessment for all devices certified by us, you will receive an EU QMS certificate. A sample of your technical documentation must be assessed, with the number of device technical documentation reviewed, depending on the risk class and total number of devices. However, for self-testing and near-patient testing devices, the sampling approach is not permitted and the technical documentation from every device must be evaluated before certificate approval.
We can also provide quality management certificates to distributors or importers carrying out any activity mentioned in points (a) and (b) of IVDR Article 16(2), subject to an application and audit procedure.
On this page
- Information for new clients
- Product risk classification & conformity assessment route
- Fees & certification cycle and surveillance
- Overview of your IVDR certification process with SGS NB 1639
- Other medical devices certification services
- Application documents
- Ancillary documents
- SGS NB 1639 additional information

Information for new clients
We are a world-leading testing, inspection, verification and certification company. To effectively meet your needs, we provide our services globally via a network of affiliates, referred to as SGS Delivering Offices. Your local SGS Delivering Office will be your primary point of contact throughout your certification process.
The following conditions apply to your CE certification:
- SGS Code of Practice
- SGS General Conditions for Certification
- Regulations Governing the Use of SGS Certification Marks
Contact your SGS Delivering Office for more information about the certification process.
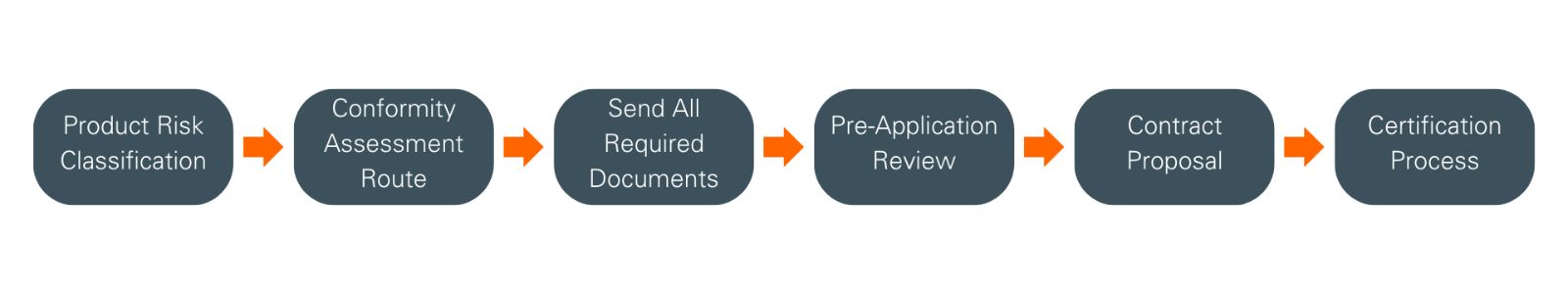
Product risk classification
The first step will be to determine your product’s classification, following the rules defined in IVDR Annex VIII. For clarification and further guidance, please refer to MDCG 2020-16.
Conformity assessment route
Depending on the device risk classification, different conformity routes may be available. We offer two conformity routes:
- Conformity assessment based on Quality Management System (QMS Audit) and Assessment of Technical Documentation (TDA), as per IVDR Annex IX
- Conformity assessment based on Production Quality Assurance, as per IVDR Annex XI
The diagram on the right shows which conformity assessment processes apply, depending on the device’s risk classification. Special considerations apply to self-testing or near-patient testing devices.
Overview of conformity assessment routes
(Acronyms: NB – Notified Body, TDA – Technical Documentation Assessment)

Fees
A list of standard fees for conformity assessment activities under the IVDR (2017/746) is available in the SGS NB 1639 Additional Information.
Contact your local SGS Delivering Office to discuss a price estimate tailored to your device portfolio.
Certification cycle and surveillance
An IVDR certificate is typically valid for five years. As stipulated in the IVDR, we reserve the right to shorten the cycle to four years or less, based on the outcomes of the conformity assessment, or due to other factors, such as vigilance issues or unannounced audit findings.
Throughout the certificate’s lifetime, we will carry out pre-arranged surveillance audits at least every 12 months to verify that your approved QMS remains effective and that certified products remain safe and perform as intended. We must also carry out at least one unannounced audit every five years.

Other medical devices certification services
We offer a broad portfolio of certification and accreditation services covering various national and international requirements. Whether your organization currently has a global reach, or if you are planning to enter additional markets, we can support your certification journey with a service tailored to your needs.
Contact your local SGS Delivering Office representative to receive further guidance on how we can help your products achieve a global reach.
Our certification services include:
- EU MDR 2017/745
- EU IVDR 2017/746
- ISO 13485 (Medical Devices QMS)
- MDSAP Program
- UKCA (UK MDR) for medical devices and in vitro diagnostics
For more information, visit the SGS Medical Devices Regulatory Compliance web page.